The Carbylamine test is employed to identify primary amines, where carbylamine is generated as a result. When primary amines are subjected to this chemical test, they yield distinct compounds in the reaction mixture. By heating aliphatic and aromatic primary amines with chloroform and ethanolic potassium hydroxide, isocyanides or carbylamines are produced, emitting an unpleasant odor. It’s important to note that this reaction doesn’t occur with secondary or tertiary amines. This test, often referred to as the carbylamine reaction or isocyanide test, is a reliable method for detecting primary amines. Another name for this reaction is Hoffmann’s isocyanide synthesis.
Hofmann’s Isocyanide Test
The carbylamine reaction serves as a specific chemical test for primary amines due to its exclusive effectiveness in their detection. This reaction is also commonly referred to as Hofmann’s isocyanide test when used for testing purposes. The procedure involves heating the test substance with chloroform and alcoholic potassium hydroxide. In the presence of a primary amine, the reaction yields isocyanide (carbylamine), which is readily identifiable by its unpleasant odor. It’s important to note that secondary and tertiary amines do not undergo the carbylamine reactions. Consequently, when subjected to the Hofmann isocyanide test, secondary and tertiary amines do not produce the foul odor associated with primary amines.
Carbylamine Reaction Mechanism
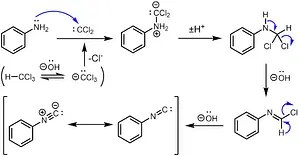
The process initiates with a dehydrogenation step, involving the removal of hydrogen halide from the provided substrate, chloroform.
This transformation yields an intermediate product known as dichlorocarbene, which is characterized by its high reactivity.
Following this, the electrophilic dichlorocarbene reacts with the nucleophilic nitrogen atom found in the primary amine.
This interaction results in the elimination of hydrochloric acid, ultimately leading to the formation of an isonitrile compound.
The carbylamine reaction can be written as –
R-NH2 + CHCl3 + 3KOH —→ RNC (Carbylamine) + 3KCl + 3H2O
How Carbylamine Reaction Work
This Reaction is primarily used to test for the presence of primary amines. Unlike secondary and tertiary amines, primary amines react uniquely in this test. Here’s how it works:
Step 1: Dehydrogenation
The reaction starts with the dehydrogenation of chloroform. During this step, hydrogen halide is removed from chloroform, giving rise to an intermediate known as dichlorocarbene. Dichlorocarbene is highly reactive and serves as a key player in this reaction.
Step 2: Attack on the Nucleophilic Nitrogen
Dichlorocarbene, being electrophilic in nature, attacks the nucleophilic nitrogen atom in the primary amine. This interaction is where the magic happens. The nucleophilic nitrogen and electrophilic dichlorocarbene form a new compound.
Step 3: Elimination of Hydrochloric Acid
The reaction isn’t complete yet. After the initial interaction, the next step involves the elimination of hydrochloric acid (HCl). This step is crucial because it ultimately leads to the formation of an isonitrile compound, which is characterized by its foul odor.
Significance of the Carbylamine Reaction
The Carbylamine Reactions is an essential tool in the chemist’s toolkit for several reasons:
Specificity: It is highly specific for primary amines, making it a reliable test to distinguish them from secondary and tertiary amines.
Versatility: The reaction can be applied to various primary amine-containing compounds, aiding in their identification.
Simple Detection: The foul odor produced during the reaction is a clear indicator of a positive result, making it easy to determine the presence of primary amines.
Crucial Reminders
- The Carbylamine reactions mechanism involves the introduction of an amine into the intermediate produced during the dehydrohalogenation of chloroform.
- This resulting intermediate is dichlorocarbene, which exhibits high reactivity.
- This reaction is alternatively called Hofmann isocyanide synthesis.
- It’s important to note that the Carbylamine reactions is not applicable for generating isocyanides from secondary and tertiary amines.
Sample Quetions:
This reaction, also known as Hoffmann’s isocyanide test, is a chemical test used to detect the presence of primary amines. It involves heating the amine with chloroform and alcoholic potassium hydroxide, resulting in the formation of foul-smelling isocyanide compounds if a primary amine is present.
The Carbylamine reaction mechanism consists of the following steps:
- Dehydrogenation of chloroform to produce dichlorocarbene.
- Attack of dichlorocarbene on the nucleophilic nitrogen of the primary amine.
- Elimination of hydrochloric acid, leading to the formation of an isonitrile compound.
The Carbylamine reaction is specific to primary amines because secondary and tertiary amines do not produce the same foul-smelling isocyanide compounds when subjected to the test. This is due to differences in the chemical reactivity of these amine groups.
The foul odor, resulting from the formation of isocyanides, is a characteristic feature of the Carbylamine reaction. It serves as a clear and immediate indicator of a positive test for primary amines, aiding in their identification.
“Hofmann’s isocyanide synthesis” is an alternative name for the Carbylamine reaction. It reflects the fact that this reaction leads to the synthesis of isocyanides from primary amines, and it is named after the chemist August Wilhelm von Hofmann, who made significant contributions to the field of organic chemistry.
Conclusion
The Carbylamine Reaction, also known as the Hoffmann’s isocyanide test, is a fascinating chemical test that serves as a valuable tool for detecting primary amines. Its simplicity, specificity, and distinctive odor-producing reaction make it a go-to method in chemical analysis. Understanding the mechanism of this reaction enhances our appreciation of its utility in the world of chemistry. So, the next time you come across this distinctive odor in the lab, you’ll know that primary amines are at play in the Carbylamine Reaction.






