How many moles of magnesium phosphate Magnesium phosphate (Mg3(PO4)2) is a compound usually used in fertilizers, food components, and as a nutritional supplement because of its important nutrients. To calculate the wide variety of moles of magnesium phosphate, you begin with its molecular weight. The molar mass of magnesium phosphate can be calculated with the aid of summing the atomic hundreds of magnesium (24.305 g/mol), phosphorus (30.974 g/mol), and oxygen (16.00 g/mol) for each atom in the compound. Once you have the molar mass, typically around 262.857 g/mol for magnesium phosphate, you can convert grams to moles the use of the formulation: moles = mass (g) / molar mass (g/mol). For instance, when you have 262.857 grams of magnesium phosphate, you would have exactly one mole. This calculation is essential in chemistry for determining quantities of materials in reactions, formulations, or dosage calculations for diverse packages, highlighting the compound’s versatility and significance in each commercial and health-related fields.
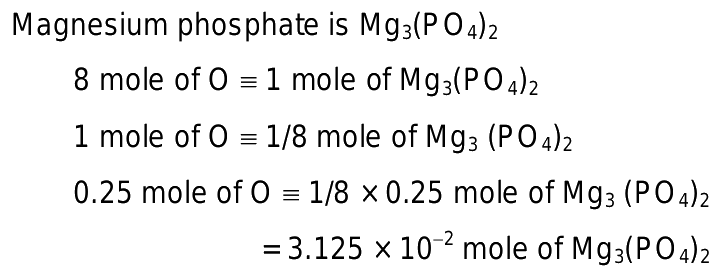
Chemical Composition of Magnesium Phosphate
- Molecular Formula: Magnesium phosphate is represented by the chemical formula Mg3(PO4)2Mg3(PO4)2Mg3(PO4)2, indicating it consists of three magnesium ions (Mg2+^{2+}2+) and two phosphate ions (PO43−PO4^{3-}PO43−).
- Elemental Components: The compound comprises magnesium (Mg), phosphorus (P), and oxygen (O) atoms. Each molecule includes three magnesium atoms, two phosphate groups, and eight oxygen atoms in total.
- Molar Mass: The molar mass of magnesium phosphate is approximately 262.86 grams per mole (g/mol). This value is derived from the atomic masses of magnesium (24.305 g/mol), phosphorus (30.974 g/mol), and oxygen (16.00 g/mol), taking into account the molecular composition.
- Chemical Structure: In the crystalline form, magnesium phosphate forms a solid compound with a specific arrangement of magnesium and phosphate ions held together by ionic bonds. This structure influences its physical properties and behavior in chemical reactions.
- Applications: Magnesium phosphate finds application in various industries, including agriculture as a fertilizer component, in medicine for its use in pharmaceuticals and dietary supplements, and in ceramics and specialty glass production due to its role as a refractory material.
Calculating Moles from Mass of Magnesium Phosphate
- Molar Mass Determination: To calculate the number of moles of magnesium phosphate ((Mg3(PO4)2), you first need to determine its molar mass. This involves summing the atomic masses of magnesium (Mg), phosphorus (P), and oxygen (O) based on their respective values from the periodic table.
- Molar Mass Calculation: The molar mass of Mg3(PO4)2Mg3(PO4)2Mg3(PO4)2 is approximately 262.86 grams per mole (g/mol). This value considers the contributions of three magnesium atoms, two phosphate groups (PO4PO4PO4), and eight oxygen atoms in total.
- Formula Application: The formula to calculate moles from mass is: Number of moles=Mass of substance (in grams)Molar mass (in g/mol)\text{Number of moles} = \frac{\text{Mass of substance(ingrams)}}{\text{Molarmass(in g/mol)}}Number of moles=Molar mass (in g/mol)Mass of substance (in grams)
- Example Calculation: If you have 200 grams of magnesium phosphate, the calculation would be Number of moles=200 g262.86 g/mol≈0.76 moles\text{Number of moles} = \frac{200 \text{ g}}{262.86 \text{ g/mol}} \approx 0.76 \text{ moles}Number of moles=262.86 g/mol200 g≈0.76 moles
- Significance in Chemistry: This calculation is fundamental for converting mass into moles, facilitating precise measurements in chemical reactions, stoichiometry, and formulation of solutions. It helps determine the quantity of substances involved and ensures accurate analysis and prediction of chemical processes.
The Role of Magnesium Phosphate in Biology and Chemistry
Magnesium phosphate (Mg3(PO4)2Mg3(PO4)2Mg3(PO4)2) plays significant roles in both biological and chemical contexts, owing to its composition and properties.
- Biological Functions: In biological systems, magnesium phosphate is crucial for cellular processes. Magnesium ions (Mg2+Mg^{2+}Mg2+) are essential cofactors for many enzymes involved in ATP (adenosine triphosphate) metabolism, DNA synthesis, and other biochemical reactions. Phosphate ions (PO43−PO4^{3-}PO43−) are integral components of DNA, RNA, and ATP, essential for energy transfer and storage within cells.
- Chemical Applications: In chemistry, magnesium phosphate finds use in various industrial and laboratory applications. It is utilized as a component in fertilizers, providing essential nutrients for plant growth and development. In pharmaceuticals, magnesium phosphate compounds are employed in medicine and dietary supplements due to their bioavailability and role in maintaining cellular function.
- Physical Properties: Magnesium phosphate exists in different forms, including crystalline structures and amorphous powders, each with specific physical and chemical properties. These properties influence its behavior in chemical reactions, its solubility in aqueous solutions, and its suitability for different applications.
- Environmental Impact: As a component of fertilizers and agricultural products, magnesium phosphate can affect soil nutrient levels and plant growth. Proper management of its application ensures sustainable agriculture practices and environmental stewardship.
- Research and Development: Ongoing research explores new applications and formulations of magnesium phosphate in fields such as materials science, ceramics, and environmental remediation. Its versatility and chemical How many moles of magnesium phosphate stability make it a valuable compound for advancing technological and scientific innovations.
Environmental Impact and Uses of Magnesium Phosphate
Magnesium phosphate (Mg3(PO4)2Mg3(PO4)2Mg3(PO4)2) serves multiple environmental and industrial purposes, reflecting its versatile properties and applications.
- Fertilizer Component: One of the primary uses of magnesium phosphate is as a component in fertilizers. It provides essential nutrients—magnesium (Mg) and phosphate (PO4)—to plants, promoting healthy growth and enhancing crop yields. Proper application helps replenish soil fertility and supports sustainable agricultural practices.
- Water Treatment: In water treatment processes, magnesium phosphate compounds are employed to remove contaminants and clarify water. They can precipitate dissolved metals and impurities, aiding in purification efforts and ensuring cleaner water supplies.
- Industrial Applications: Magnesium phosphate finds application in various industrial sectors. It is utilized in ceramics and glass manufacturing due to its role as a refractory material, contributing to the production of heat-resistant and durable products. Additionally, it serves as a catalyst in chemical reactions and as a stabilizer in pharmaceutical formulations.
- Environmental Impact: The environmental impact of magnesium phosphate is carefully managed to minimize adverse effects. Controlled use in agriculture helps prevent nutrient runoff into water bodies, which can otherwise lead to eutrophication and ecological imbalances. Efforts focus on optimizing application methods to enhance efficiency and reduce environmental footprint.
- Research and Development: Ongoing research explores novel uses and formulations of magnesium phosphate. This includes its potential applications in environmental remediation, such as soil conditioning and remediation of contaminated sites, highlighting its role in sustainable development practices.
Conclusion
In conclusion, magnesium phosphate How many moles of magnesium phosphate (Mg3(PO4)2 plays a crucial role across various sectors, from agriculture to industry and environmental management. Its significance lies in its dual capacity as a nutrient source in fertilizers, supporting crop growth, and as a versatile compound in industrial processes, including water treatment and ceramic production. The compound’s environmental impact is carefully monitored, emphasizing sustainable practices to mitigate potential risks while maximizing its beneficial applications. Ongoing research continues to explore new uses and innovations, underscoring magnesium phosphate’s potential in addressing contemporary challenges in agriculture, water quality management, and materials science. As such, magnesium phosphate remains integral to advancements in technology and environmental stewardship, contributing to a more sustainable future.
FAQs
Q: 1What is the chemical formula for magnesium phosphate?
Ans:The chemical formula for magnesium phosphate is Mg3(PO4)2Mg3(PO4)2Mg3(PO4)2. It consists of three magnesium ions (Mg2+^{2+}2+) and two phosphate ions (PO43−PO4^{3-}PO43−).
Q:2 Why is magnesium phosphate important in agriculture?
Ans: Magnesium phosphate is used in fertilizers to provide essential nutrients—magnesium and phosphate—to plants. These nutrients support plant growth and enhance crop yields, contributing to agricultural productivity.
Q:3 Can magnesium phosphate be found naturally?
Ans Yes, magnesium phosphate can occur naturally in various forms, including minerals and rock deposits. These natural sources are sometimes used as raw materials in industrial processes or as sources of agricultural fertilizers.






