pH full form “potential of hydrogen“ refers to the concentration of hydrogen ions (H+) in a solution, which serves as the foundation of the pH scale. The pH scale is a logarithmic scale that quantifies a solution’s acidity or alkalinity depending on the concentration of hydrogen ions. The term “potential of Hydrogen” refers to the role of hydrogen ions in determining the pH of a solution in the past.
What is pH full form ?
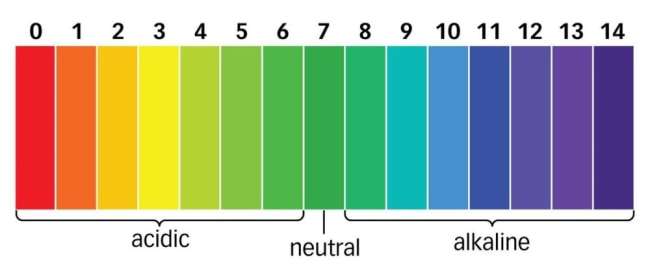


The full form of pH is “potential of Hydrogen.” pH is a measure of the acidity or alkalinity of a solution and is based on the concentration of hydrogen ions (H+) present in the solution. It is a logarithmic scale that ranges from 0 to 14, with pH 7 considered neutral, pH less than 7 indicating acidity, and pH greater than 7 indicating alkalinity. The pH scale is widely used in various fields, including chemistry, biology, environmental science, and medicine, to quantify the acidity or alkalinity of substances and solutions.
Importance of pH full form
Biological Functioning: pH is critical for the functioning of enzymes and biochemical reactions in residing organisms. Many enzymes function optimally at precise pH degrees, and deviations can restrict metabolic processes.
Soil Health: In agriculture, soil pH impacts nutrient availability for plant life. Certain vitamins grow to be more or less to be had relying at the pH level, influencing plant growth and crop yields.
Aquatic Ecosystems: pH performs a vital position in preserving the fitness of aquatic environments. Fish and other aquatic organisms have precise pH tiers wherein they thrive; deviations can lead to pressure or demise.
Water Quality: Monitoring pH is crucial in water remedy methods to make certain safe consuming water and the effectiveness of disinfection methods. It also allows save you corrosion in plumbing systems.
Food Safety and Quality: pH influences meals protection, flavor, and texture. Many meals renovation strategies, inclusive of pickling, rely upon acidic situations to inhibit bacterial increase.
Industrial Processes: Many chemical reactions in manufacturing and enterprise are sensitive to pH. Maintaining an appropriate pH can optimize production procedures, product high-quality, and safety.
Medical Diagnosis: pH ranges are crucial in scientific diagnostics. For instance, blood pH is an important indicator of metabolic and respiration fitness, and abnormalities can signify underlying health troubles.
Role of pH full form
Enzymatic Activity: pH regulates the pastime of enzymes, which might be critical for biochemical reactions in living organisms. Each enzyme has an most beneficial pH range, and deviations can lead to reduced performance or denaturation.
Nutrient Availability: In agriculture, soil pH determines the provision of nutrients to flora. Certain nutrients, like iron, are more handy at lower pH stages, while others, like phosphorus, are extra available at better pH levels.
Aquatic Life Support: pH levels are vital for the survival of aquatic organisms. Many fish and aquatic species have precise pH tolerances; keeping optimum pH is critical for their health and reproduction.
Corrosion Control: In engineering and creation, pH performs a role in corrosion fees of metals. Monitoring and adjusting pH can help save you rusting and degradation of infrastructure.
Fermentation Processes: In meals production, pH is a key issue in fermentation, affecting taste, texture, and upkeep. For instance, decrease pH degrees in yogurt manufacturing inhibit pathogenic bacteria and promote the increase of beneficial micro organism.
Pharmaceutical Formulation: In prescription drugs, pH impacts drug solubility, balance, and absorption. Many medicines require precise pH conditions to be effective while administered.
Diagnostic Indicator: In clinical settings, pH measurements (like blood pH) are critical diagnostic signs. Abnormal pH degrees can signal diverse scientific situations, along with respiration and metabolic issues.
Practical uses of pH full form
| Field | Practical Uses of pH |
|---|---|
| Agriculture | – Soil pH testing to determine nutrient availability for crops. – Adjusting soil pH with lime (to raise) or sulfur (to lower) for optimal plant growth. |
| Aquaculture | – Monitoring water pH to maintain healthy environments for fish and other aquatic organisms. – Adjusting pH to prevent stress or disease in farmed species. |
| Water Treatment | – Testing and adjusting pH levels in drinking water to ensure safety and compliance with health standards. – Preventing corrosion in pipes and fixtures. |
| Food Industry | – Controlling pH during fermentation processes (e.g., yogurt, cheese) for flavor and preservation. – Using acidic pH to inhibit microbial growth in food preservation (e.g., pickling). |
| Pharmaceuticals | – Formulating drugs with specific pH levels for optimal solubility and absorption. – Adjusting pH in intravenous solutions for patient safety. |
| Environmental Science | – Measuring pH levels in soil and water bodies to assess pollution and ecosystem health. – Studying the effects of acid rain on natural environments. |
| Chemical Manufacturing | – Controlling pH in industrial processes to optimize reaction rates and product quality. – Using pH meters in quality control to ensure consistent product characteristics. |
Limitations of pH full form
Narrow Measurement Range: The pH scale usually tiers from 0 to 14, which might not competently constitute extraordinarily acidic or primary situations discovered in certain commercial processes or specialised medical packages.
Temperature Sensitivity: pH readings may be stricken by temperature fluctuations. Most pH meters need temperature reimbursement to ensure correct readings, complicating measurements in variable conditions.
Electrode Limitations: pH electrodes can degrade over time, main to misguided readings. They require everyday calibration and renovation, which can be time-ingesting and pricey.
Sample Contamination: pH measurements can be inspired by using contaminants within the sample, consisting of oils, suspended solids, or other chemicals, main to inaccurate outcomes.
Buffer Solutions: The presence of buffer answers can have an effect on pH readings, as buffers resist adjustments in pH and can mask the authentic acidity or alkalinity of the solution.
Complex Solutions: In complicated combos (like biological fluids or industrial waste), a couple of elements can affect pH, making it difficult to interpret the outcomes with out extra analyses.
PH Mismatch: The ideal pH for one application may not be suitable for another. For example, at the same time as a low pH is beneficial for food preservation, it may be damaging to certain biological strategies or aquatic existence.
Significance of pH full form
| Field | Significance of pH |
|---|---|
| Biology | – Critical for enzymatic activity and metabolic processes. – Influences cellular function and overall organism health. |
| Agriculture | – Determines nutrient availability in soil, affecting crop yields. – Guides soil management practices for optimal plant growth. |
| Aquatic Ecosystems | – Essential for the survival and reproduction of aquatic species. – Influences biodiversity and ecosystem stability. |
| Water Quality | – Key parameter in assessing the safety and quality of drinking water. – Affects the effectiveness of water treatment processes. |
| Food Preservation | – Helps control microbial growth in food products, extending shelf life. – Influences flavor and texture in fermented foods. |
| Pharmaceuticals | – Affects drug formulation, stability, and bioavailability. – Essential for ensuring patient safety in medical treatments. |
| Environmental Monitoring | – Important for assessing pollution levels and ecosystem health. – Used in evaluating the impact of acid rain and other environmental changes. |
Value of pH full form
The pH scale is a logarithmic scale that measures the acidity or basicity of a solution. The pH scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are basic.
The value of pH is calculated using the following formula:
pH = -log([H+])where [H+] is the concentration of hydrogen ions in the solution.
- A pH of 7 is neutral, meaning that the solution has equal amounts of hydrogen and hydroxide ions.
- A pH less than 7 is acidic, meaning that the solution has more hydrogen ions than hydroxide ions.
- A pH greater than 7 is basic, meaning that the solution has more hydroxide ions than hydrogen ions.
The pH of a solution can be affected by a number of factors, including the type of acid or base present, the concentration of the acid or base, and the temperature of the solution.
Examples of pH full form
Here are some examples of the pH of common substances:
- Pure water: 7
- Lemon juice: 2
- Milk of magnesia: 10
- Battery acid: 0
The pH of a solution is an important indicator of its acidity or basicity. This can be important for a number of reasons, including:
- The pH of a solution can affect the solubility of other substances in the solution.
- The pH of a solution can affect the biological activity of the solution.
- The pH of a solution can affect the corrosion of metals.
Scale of pH full form
| pH Value | Acidity/Alkalinity |
|---|---|
| 0 | Strong Acid |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | Weak Acid |
| 6 | |
| 7 | Neutral |
| 8 | |
| 9 | |
| 10 | Weak Alkali |
| 11 | |
| 12 | |
| 13 | |
| 14 | Strong Alkali |
Here is a more detailed explanation of the pH scale:
- 0 to 6: These are acidic solutions. The lower the pH, the more acidic the solution.
- 7: This is a neutral solution. It has equal amounts of hydrogen and hydroxide ions.
- 8 to 14: These are basic solutions. The higher the pH, the more basic the solution.
Conclusion
(pH) refers to the measurement of the concentration of hydrogen ions (H+) in a solution. The pH scale, based on this measurement, is a logarithmic scale that indicates the acidity or alkalinity of a solution. The term “potential of Hydrogen” is a historical reference to the role of hydrogen ions in determining the pH of a solution and has become a widely used concept in various scientific fields, such as chemistry, biology, and environmental science, for assessing and characterizing the acidity or alkalinity of substances.
FAQ's
Q1: What does pH full form?
A: pH stands for “potential of hydrogen,” which refers to the concentration of hydrogen ions in a solution.
Q2: What is the pH scale?
A: The pH scale ranges from 0 to 14. A pH of 7 is considered neutral, below 7 is acidic, and above 7 is basic (alkaline).
Q3: How is pH measured?
A: pH can be measured using pH indicators (like litmus paper), pH meters, or pH test strips that change color based on the acidity or alkalinity of the solution.
Q4: Why is pH important in agriculture?
A: pH affects soil nutrient availability. Certain nutrients are more accessible at specific pH levels, influencing plant growth and crop yields.
Q5: How does pH affect aquatic life?
A: Most aquatic organisms have specific pH tolerances. Deviations from optimal pH levels can lead to stress, disease, or death in fish and other aquatic species.






