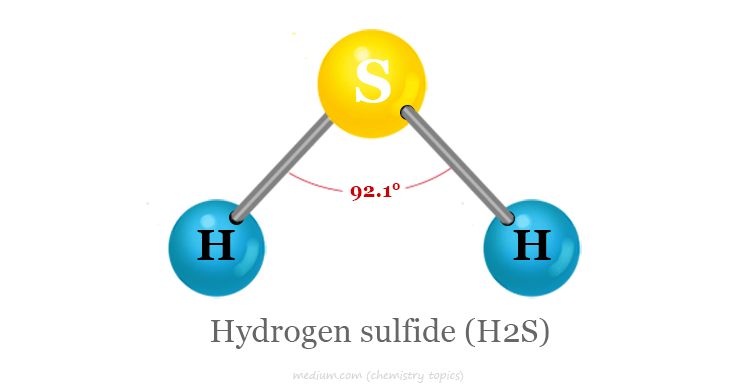
How many atoms are present in h2s molecule A hydrogen sulfide molecule, represented by means of the chemical method H2S, contains 3 individual atoms. Specifically, this molecule carries two hydrogen atoms and one sulfur atom. Hydrogen sulfide is a drab gasoline, acknowledged for its distinct foul smell similar to that of rotten eggs. It takes place clearly in volcanic gases and some mineral waters, in addition to during the bacterial breakdown of natural depend inside the absence of oxygen, including in swamps and sewers. This compound additionally seems as a byproduct in many commercial processes inclusive of petroleum refining and herbal gas extraction. Structurally, the 2 hydrogen atoms are every covalently bonded to the vital sulfur atom, forming a dishonest molecular geometry just like that of water (H2O), but with bond angles slightly adjusted due to the differing electron requirements of sulfur in comparison to oxygen. This simple molecular composition performs a vital function in diverse biological and environmental procedures, making it a topic of look at in more than one fields of technological know-how.
How many atoms are present in h2s molecule
Here is a detailed breakdown in 10 points regarding the number of atoms in a molecule of hydrogen sulfide (H2S):
- Molecular Formula: H2S.
- Total Atoms: The molecule comprises three atoms.
- Hydrogen Atoms: It includes two hydrogen (H) atoms.
- Sulfur Atom: It contains one sulfur (S) atom.
- Covalent Bonds: Each hydrogen atom is covalently bonded to the sulfur.
- Molecular Geometry: The arrangement forms a bent structure.
- Electron Sharing: The bonding involves sharing of electrons between sulfur and hydrogen.
- Molecular Composition: This composition results in a simple binary compound.
- Occurrence: H2S is commonly found in natural gas, volcanic gases, and as a byproduct of organic decay.
- Role and Relevance: Despite its toxicity and foul smell, it plays significant roles in various environmental and biological processes.
How to count atoms in a molecule
Counting the number of atoms in a molecule can be done by following these step-by-step guidelines:
- Identify the Molecular Formula: Start by looking at the molecular formula of the compound. This formula tells you which elements are present and how many atoms of each are in a single molecule.
- List Each Element: Write down each element (chemical symbol) from the formula.
- Count Atoms of Each Element: Next to each element, write the number of atoms indicated by the subscript in the molecular formula. If there is no subscript next to an element, it means there is one atom of that element.
- Consider Polyatomic Groups: If the molecule includes a polyatomic ion or group (like OH, NH4, etc.), remember that the subscript outside the parentheses multiplies the number of atoms of each element inside the parentheses.
- Total Atoms Calculation: Add up the total number of atoms of all elements in the molecule.
- Example – Water (H2O):
- H (Hydrogen) = 2 atoms
- O (Oxygen) = 1 atom
- Total atoms = 2 + 1 = 3
7. Example – Ammonium Sulfate ( (NH4)2SO4):
- N (Nitrogen) = 2 atoms (because NH4 is multiplied by 2)
- H (Hydrogen) = 8 atoms (4 hydrogen atoms per NH4, times 2)
- S (Sulfur) = 1 atom
- O (Oxygen) = 4 atoms
- Total atoms = 2 + 8 + 1 + 4 = 15
8. Complex Molecules: For more complex molecules or those not clearly showing subscripts (often seen in organic compounds), it may help to draw the molecule or use a molecular model kit.
9. Using Software Tools: For very large or complex molecules, chemical drawing software or online molecule editors can be used to visualize and automatically count atoms.
10. Continuous Learning: Understanding basic chemical nomenclature and structures helps greatly in correctly interpreting molecular formulas and thus counting atoms accurately.
How H2S is used in industries
Hydrogen sulfide (H2S) is a colorless, toxic gas with a characteristic odor of rotten eggs, but despite its toxicity and unpleasant smell, it finds several industrial uses. Here are some of the ways in which H2S is utilized across various industries:
- Oil and Gas Industry: H2S is a common byproduct in the oil and gas industry, especially during the processing of crude oil and natural gas. It must be removed from these products before they can be used. The process of removing H2S is known as “sweetening.” Moreover, the sulfur that is recovered from the desulfurization process is used to produce sulfuric acid and other sulfur compounds, which are valuable industrial and chemical commodities.
- Chemical Production: H2S is used in the chemical industry to manufacture sulfur and sulfuric acid. Sulfuric acid is one of the most important industrial chemicals and is used in the production of fertilizers, mineral processing, petroleum refining, and chemical synthesis.
- Production of Metal Sulfides: H2S is used in the production of metal sulfides, which are used in various applications including photovoltaics, photonics, and as catalysts. Metal sulfides are made by reacting H2S with metal oxides.
- Leather Processing: Hydrogen sulfide is used in the tanning industry. It can be used to help remove hair and other keratin-containing materials from animal hides in preparation for the tanning process.
- Agricultural Applications: In agriculture, controlled amounts of H2S can be used as an agricultural disinfectant and pesticide. It helps in controlling the growth of bacteria and fungi.
- Environmental Monitoring and Analysis: Due to its distinct smell and toxicity, H2S is often monitored as an indicator of environmental pollution, particularly in industrial settings and wastewater treatment facilities. It is important to track H2S levels to ensure worker safety and compliance with environmental regulations.
- Water Treatment: Hydrogen sulfide can be used to remove heavy metals from water through the formation of insoluble metal sulfides, aiding in water purification processes.
- Pharmaceuticals: Research is ongoing into the potential use of H2S in pharmaceuticals. It is being studied for its possible protective effects in various medical conditions, including cardiovascular diseases and strokes, as it may mimic the beneficial effects of dietary restriction.
- Analytical Reagent: H2S is used in analytical chemistry as a reagent for the qualitative determination of metal ions.
- Mercaptan Synthesis: It is also used in the synthesis of organic sulfur compounds known as mercaptans, which are subsequently used in a wide range of applications, including as additives in the oil and gas industry.
Common reactions and products
Hydrogen sulfide (H2S) is a reactive compound involved in various chemical reactions, with the resulting products having important industrial and environmental significance. Here are some common reactions involving H2S and their respective products:
1.Combustion:
- Reaction: H2S + O2 → SO2 + H2O
- Products: Sulfur dioxide (SO2) and water (H2O). This is a typical combustion reaction, where hydrogen sulfide burns in the presence of oxygen to form sulfur dioxide and water. Sulfur dioxide is a significant pollutant and is also an intermediate in the production of sulfuric acid.
2. Oxidation to Sulfuric Acid:
- Reaction: 2 H2S + 3 O2 → 2 H2O + 2 SO2 (first step); 2 SO2 + O2 → 2 SO3 (second step); SO3 + H2O → H2SO4
- Products: Sulfuric acid (H2SO4). This multi-step process is fundamental in industries to convert hydrogen sulfide into sulfuric acid, which is one of the most important industrial chemicals.
3. Reduction of Metal Oxides:
- Reaction: H2S + MO (where M is a metal) → MS + H2O
- Products: Metal sulfide (MS) and water (H2O). This reaction is used in the metallurgical industry to produce metal sulfides, which are useful in various applications including semiconductors and batteries.
4. Formation of Sulfides in Organic Synthesis:
- Reaction: R-X + NaSH → R-SH + NaX (where R is an organic group, and X is a halide)
- Products: Thiol (R-SH) and a sodium halide (NaX). This reaction is utilized in organic synthesis for introducing thiol groups into molecules.
5. Acid-Base Reaction with Strong Bases:
- Reaction: H2S + 2 NaOH → Na2S + 2 H2O
- Products: Sodium sulfide (Na2S) and water (H2O). Sodium sulfide is used in various industrial processes, including as a part of the Kraft process in paper manufacturing.
6. Claus Process (Recovery of Elemental Sulfur):
- Reaction: 2 H2S + SO2 → 3 S + 2 H2O
- Products: Elemental sulfur (S) and water (H2O). This reaction is crucial in the oil and gas industry to convert hydrogen sulfide from natural gas into elemental sulfur, which can be sold commercially.
7. Precipitation of Heavy Metals:
- Reaction: H2S + M^2+ → MS + 2 H^+
- Products: Metal sulfide (MS) and hydrogen ions (H^+). This reaction is used in wastewater treatment to remove heavy metals by forming insoluble metal sulfides.
Conclusion
In end, How many atoms are present in h2s molecule is a multifaceted chemical compound that, in spite of its toxicity and smelly odor, plays a critical function across diverse industrial sectors. Its capacity to undergo various chemical reactions permits its use within the manufacturing of critical materials which include sulfuric acid, elemental sulfur, and various metallic sulfides. These substances are integral to procedures inside the chemical production, metallurgical, environmental management, or even pharmaceutical industries.
The reactions related to H2S aren’t simplest essential for synthesizing precious business chemical substances but also for environmental pollution control, mainly in treating waste streams and getting better treasured substances from commercial by using-merchandise. However, the hazardous nature of H2S demands stringent protection protocols and monitoring to save you its damaging health and environmental affects.
FAQs
Q: 1How many atoms are in an H2S molecule?
Ans:: An H2S molecule contains a total of three atoms: two hydrogen atoms (H) and one sulfur atom (S).
Q: 2 Why does an H2S molecule have three atoms?
Ans: The chemical formula H2S indicates that there are two atoms of hydrogen bonded to one atom of sulfur, resulting in a total of three atoms in the molecule.
Q:3 How do you count the atoms in an H2S molecule?
Ans: To count the atoms in an H2S molecule, you simply add up the number of each type of atom indicated by the molecular formula: 2 hydrogen atoms and 1 sulfur atom.
Q: 4 What is the significance of the number of atoms in an H2S molecule?
Ans: Understanding the number of atoms in an H2S molecule is important for various applications in chemistry, industry, and environmental science. It helps in predicting the molecule’s properties and behavior in reactions.






