How to calculate molecular mass Calculating the molecular mass of a compound involves adding up the atomic masses of all the atoms present in its molecular formula. Each element contributes to the total molecular mass based on its atomic weight, which is found on the periodic table. To compute it, multiply the atomic mass of each element by the number of atoms of that element in the molecule and then sum these values. For instance, in water (H₂O), hydrogen has an atomic mass of approximately 1.008 atomic mass units (amu) and oxygen about 16.00 amu. Thus, the molecular mass of water is calculated as (2 × 1.008 amu for hydrogen) + (1 × 16.00 amu for oxygen) = 18.016 amu. This method is essential in chemistry for determining molecular structures, understanding chemical reactions, and analyzing substances in scientific research and industry.
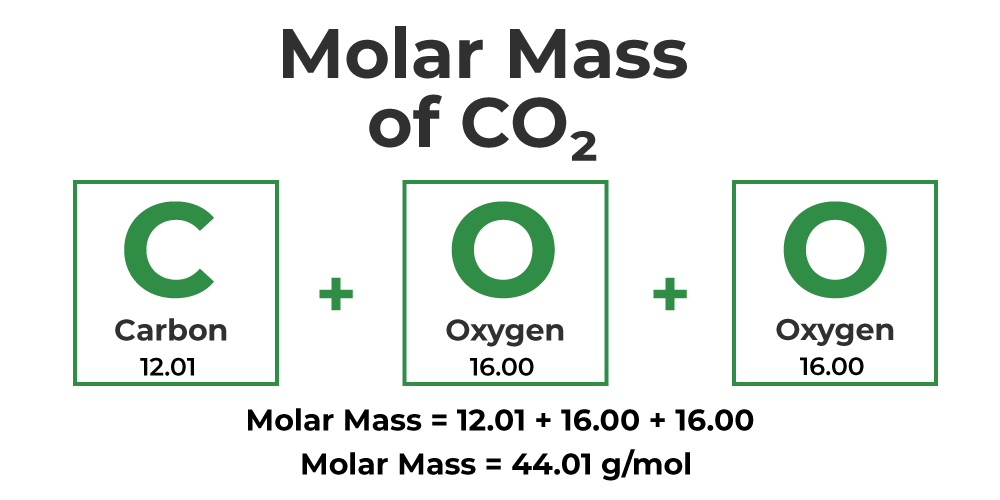
Methods to Calculate Molecular Mass
- Sum of Atomic Masses:
- Identify the molecular formula and sum the atomic masses of all atoms present. Use the atomic masses from the periodic table, typically measured in atomic mass units (amu) or grams per mole (g/mol).
2. Example Calculation:
- For a molecule like water (H₂O), calculate by multiplying the atomic mass of each element (hydrogen and oxygen) by the number of atoms present (2 for hydrogen and 1 for oxygen), then summing these values.
3. Using Molecular Formula:
- Ensure accuracy by correctly identifying and counting atoms in the molecular formula. This step is crucial as errors can lead to incorrect molecular mass calculations.
4. Application in Chemistry:
- Molecular mass calculations are fundamental in chemistry for determining stoichiometry in reactions, understanding molecular structures, and predicting physical properties such as boiling points and solubility.
5. Molar Mass Calculation:
- Convert the molecular mass into molar mass by expressing it in grams per mole (g/mol), which is useful for quantitative analysis and measurements in chemical experiments.
Molecular Mass of Compounds
- Definition:
- The molecular mass of a compound is the sum of the atomic masses of all atoms in its molecular formula. It is expressed in atomic mass units (amu) or grams per mole (g/mol).
2. Calculation Method:
- Identify the molecular formula of the compound and determine the number of each type of atom present.
- Look up the atomic masses of each element from the periodic table.
- Multiply the atomic mass of each element by the number of atoms of that element in the molecule.
- Sum these values to find the total molecular mass.
3. Example:
- For water (H₂O), calculate the molecular mass as:
- Atomic mass of hydrogen (H): ~1.008 amu × 2 atoms = ~2.016 amu
- Atomic mass of oxygen (O): ~16.00 amu × 1 atom = ~16.00 amu
- Total molecular mass of water = ~18.016 amu
4. Importance in Chemistry:
- Molecular mass is crucial for determining the stoichiometry of chemical reactions, understanding molecular structures, predicting physical and chemical properties, and analyzing substances in scientific research and industry.
5. Application:
- Used extensively in fields such as pharmaceuticals, materials science, environmental science, and biochemistry for designing molecules, studying molecular interactions, and developing new materials and compounds.
How to calculate molecular mass
- Identify the Molecular Formula: Determine the chemical formula of the compound, which specifies the types and numbers of atoms present.
- Find Atomic Masses: Look up the atomic masses of each element in the compound from the periodic table. Atomic masses are typically given in atomic mass units (amu) or grams per mole (g/mol).
- Multiply and Sum: Multiply the atomic mass of each element by the number of atoms of that element in the compound as indicated by the subscript in the molecular formula.
4. Example Calculation: For water (H₂O):
- Hydrogen (H) atomic mass ≈ 1.008 amu
- Oxygen (O) atomic mass ≈ 16.00 amu
- Molecular mass of water = (2 × 1.008 amu for H) + (1 × 16.00 amu for O)
- Molecular mass of water ≈ 18.016 amu
5. Units: The result is typically expressed in atomic mass units (amu) or grams per mole (g/mol), depending on the context and application.
Formulas and Calculations
Formulas and calculations play a crucial role in chemistry for determining various quantities and properties. Here are key aspects:
- Molecular Mass Calculation:
- Formula: Molecular mass (𝑀M) is calculated by summing the atomic masses (𝑚m) of all atoms in a molecule: 𝑀=𝑚1+𝑚2+𝑚3+…M=m1+m2+m3+… Where 𝑚1,𝑚2,𝑚3,…m1,m2,m3,… are the atomic masses of the atoms present in the molecule.
2. Molar Mass Calculation:
Formula: Molar mass (𝑀molarMmolar) is the mass of one mole of a substance. It is numerically equal to its molecular mass but expressed in grams per mole (g/mol): 𝑀molar=𝑀molecularMmolar=Mmolecular For example, the molar mass of water (H₂O) is approximately 18.016 g/mol.
3. Stoichiometry in Reactions:
- Formulas: Stoichiometry involves using balanced chemical equations to determine the quantities of reactants and products in chemical reactions. This helps predict yields, determine limiting reactants, and calculate reaction efficiencies.
4. Concentration Calculations:
- Formulas: Concentration of solutions is expressed in various units such as molarity (M), molality (m), and normality (N). Calculations involve relating the amount of solute to the volume or mass of solvent or solution.
5. Energy Calculations:
Formulas: Thermodynamic calculations involve formulas such as those for heat (q), enthalpy (Δ𝐻ΔH), entropy (Δ𝑆ΔS), and Gibbs free energy (Δ𝐺ΔG). These formulas are fundamental for understanding energy changes in chemical reactions and processes.
Conclusion
In conclusion, How to calculate molecular mass formulas and calculations form the backbone of chemical analysis and understanding in chemistry. From calculating molecular masses and molar masses to determining stoichiometry in reactions and concentrations in solutions, these mathematical tools provide precise methods for quantifying substances and predicting their behavior. Beyond basic measurements, formulas extend into thermodynamics, guiding assessments of energy changes in reactions. Mastery of these calculations enables scientists to elucidate molecular structures, optimize chemical processes, and innovate in fields ranging from pharmaceuticals to materials science. By leveraging formulas and calculations, researchers and industry professionals alike can advance knowledge, enhance efficiency, and address complex challenges in the pursuit of scientific discovery and technological innovation.
FAQs
Q: 1.What is molecular mass?
Ans::Molecular mass refers to the sum of the atomic masses of all atoms in a molecule. It is expressed in atomic mass units (amu) or grams per mole (g/mol).
Q: 2How do you calculate molecular mass?
Ans: To calculate molecular mass, multiply the atomic mass of each element in the molecule by the number of atoms of that element, as indicated by the subscript in the molecular formula. Then, sum these values to find the total molecular mass.
Q:3Why is molecular mass important in chemistry?
Ans: Molecular mass is crucial for determining the composition of substances, predicting their properties, and quantifying reactants and products in chemical reactions. It provides essential information for understanding molecular structures and behaviors.
Q:4What units are used for molecular mass?
Ans:Molecular mass is typically expressed in atomic mass units (amu) or grams per mole (g/mol), depending on the context and application.






