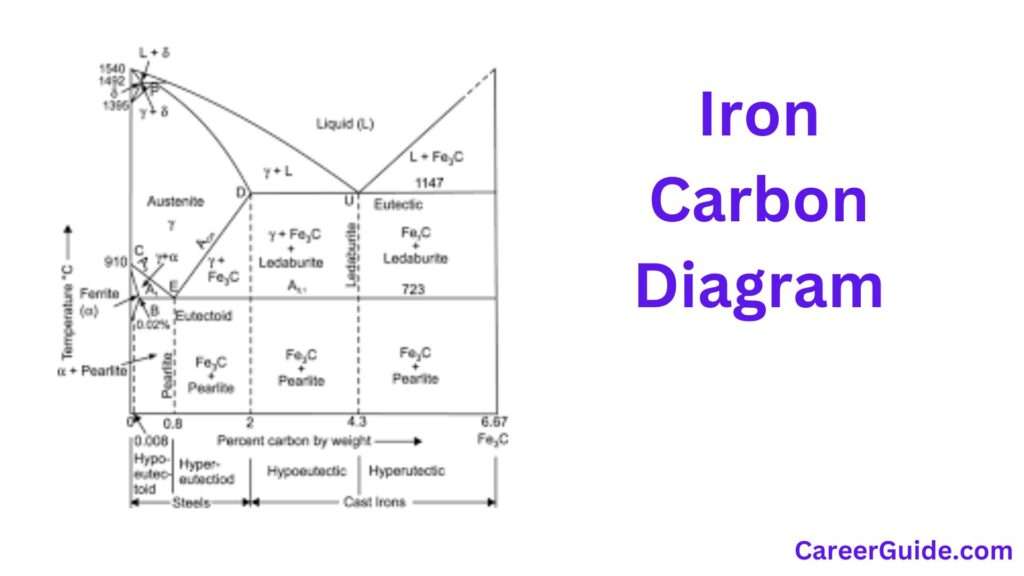
The Iron-Carbon diagram, also known as the Fe-C phase diagram, is a fundamental graphical representation in metallurgy. It illustrates the phases and transformations of iron-carbon alloys at various carbon concentrations and temperatures. This diagram is crucial for understanding the behavior of steel and cast iron during heating and cooling, revealing the stability regions of different phases such as ferrite, austenite, and cementite. The diagram is characterized by key features such as eutectoid, eutectic, and peritectic points, which indicate significant phase transformations.
- Fundamentals of the Iron Carbon Diagram
- Phases and Microstructures inIron Carbon Diagram Alloys
- Understanding the Eutectoid Reaction in Iron Carbon Diagram
- The Eutectic Reaction and Cast Irons in Iron Carbon Diagram
- The Peritectic Reaction in Steel Making in Iron Carbon Diagram
- Heat Treatment of Steel in Iron Carbon Diagram
- Transformation Iron Carbon Diagram: TTT and CCT
- Influence of Alloying Elements in Iron Carbon Diagram
- FAQ
Fundamentals of the Iron Carbon Diagram
The Iron-Carbon Phase Diagram
The Iron-Carbon section diagram is a graphical illustration of the diverse levels and their ameliorations in iron-carbon alloys. It is split into unique regions, every representing a awesome section or a aggregate of levels that arise at unique temperatures and carbon concentrations. The diagram generally covers carbon contents as much as 6.67%, which corresponds to the composition of cementite (Fe₃C). The vital capabilities of the diagram consist of the liquidus and solidus lines, which delineate the bounds among liquid and stable levels, and diverse section limitations that imply section modifications together with the transformation from austenite to pearlite.
Key Phases: Ferrite, Austenite, Cementite
Ferrite (α-Fe):
Ferrite is a body-targeted cubic (BCC) shape and may dissolve a small quantity of carbon, commonly as much as 0.02% at room temperature. It is soft, ductile, and has a exceedingly low tensile strength. Ferrite is magnetic and bureaucracy the matrix in low-carbon steels.
Austenite (γ-Fe):
Austenite is a face-targeted cubic (FCC) shape that may dissolve drastically extra carbon than ferrite, as much as 2.14% at 1147°C. It is non-magnetic and has a better density and decrease ductility in comparison to ferrite. Austenite is solid best at excessive temperatures and transforms to different levels upon cooling.
Cementite (Fe₃C):
Cementite is an iron carbide with a complicated orthorhombic shape. It is a tough and brittle compound that drastically will increase the hardness of metallic. Cementite is found in pearlite, bainite, and the shape of white forged iron.
Eutectoid, Eutectic, and Peritectic Reactions
Eutectoid Reaction:
This response takes place at 727°C and 0.76% carbon. During this response, austenite decomposes right into a great aggregate of ferrite and cementite, forming pearlite. The eutectoid response is vital in metallic manufacturing because it determines the microstructure and mechanical residences of steels.
Eutectic Reaction:
The eutectic response takes place at 1147°C and 4.3% carbon. At this factor, liquid iron solidifies right into a aggregate of austenite and cementite simultaneously. This response is important withinside the formation of forged irons, in particular in reaching the preferred microstructure and residences of various varieties of forged iron.
Peritectic Reaction:
The peritectic response takes place at 1495°C and 0.18% carbon. In this response, liquid iron and ferrite integrate to shape austenite. The peritectic factor is much less not unusualplace in sensible metallurgy however performs a position withinside the solidification technique of sure metallic grades, influencing their very last shape and residences.
Phases and Microstructures inIron Carbon Diagram Alloys
Pure Iron Phases
Pure iron, whilst loose from carbon and different alloying elements, famous numerous wonderful levels relying on temperature:
Alpha Iron (α-Fe) or Ferrite:
Ferrite has a body-focused cubic (BCC) shape and stays solid as much as temperatures of round 912°C. It is characterised via way of means of its softness and ductility, with a completely constrained capacity to dissolve carbon (as much as about 0.02%). Ferrite is magnetic and is a enormous element in low-carbon steels, wherein it affords proper ductility and toughness.
Gamma Iron (γ-Fe) or Austenite:
Austenite functions a face-focused cubic (FCC) shape and is solid among about 912°C and 1394°C. This section can dissolve a large amount of carbon, that is important for developing excessive-energy steels. Austenite is non-magnetic and has a better density in comparison to ferrite, contributing to the hardness and energy of steels.
Delta Iron (δ-Fe):
Delta iron additionally possesses a BCC shape and is solid above 1394°C as much as the melting factor of natural iron, round 1538°C. It resembles ferrite however happens at plenty better temperatures and performs a function withinside the solidification of steels.
Fe-C Phases: Ferrite, Austenite, Cementite
Ferrite (α-Fe):
Ferrite is a BCC section with very constrained carbon solubility. It bureaucracy the matrix in lots of steels and affords enormous ductility. Ferrite is the number one section in low-carbon steels and is found in microstructures consisting of pearlite and bainite.
Austenite (γ-Fe):
Austenite, with its FCC shape, can dissolve a good sized quantity of carbon (as much as 2.14% at 1147°C). It is important in lots of warmness remedy processes, consisting of quenching and tempering, wherein it transforms into different levels like martensite or pearlite primarily based totally on cooling rates.
Cementite (Fe₃C):
Cementite is a difficult and brittle iron carbide that looks in numerous microstructures inclusive of pearlite and bainite. It will increase the hardness and put on resistance of metal and solid iron.
Pearlite, Bainite, and Martensite
Pearlite:
Pearlite is a lamellar combination of ferrite and cementite that bureaucracy via the eutectoid response at round 727°C and 0.76% carbon. It includes alternating layers of soft, ductile ferrite and difficult, brittle cementite. Pearlite gives a stability of energy and ductility and is normally located in lots of carbon steels.
Bainite:
Bainite bureaucracy at temperatures decrease than the ones for pearlite however above the temperature at which martensite begins offevolved to form. It may be categorized into top bainite and decrease bainite, primarily based totally on transformation temperature. Upper bainite functions ferrite and elongated cementite, even as decrease bainite shows a needle-like shape. Bainite affords a terrific aggregate of energy and toughness, making it appropriate for excessive-overall performance applications.
Martensite:
Martensite is produced via way of means of the speedy quenching of austenite, ensuing in a supersaturated, body-focused tetragonal (BCT) shape. This section may be very difficult and brittle because of the trapped carbon atoms in the iron lattice. Martensite imparts excessive hardness and energy to quenched steels, aleven though it usually calls for tempering to lessen brittleness and obtain favored mechanical properties.
Understanding the Eutectoid Reaction in Iron Carbon Diagram
Definition and Importance
The eutectoid response is a vital transformation withinside the iron-carbon machine that takes place at a selected composition and temperature. It includes the transformation of a unmarried segment into awesome stages upon cooling. In the case of iron-carbon alloys, the eutectoid response takes place at a temperature of about 727°C and a carbon awareness of 0.76%. During this response, austenite (γ-Fe) transforms right into a combination of ferrite (α-Fe) and cementite (Fe₃C), referred to as pearlite.
Formation of Pearlite
Pearlite is the made from the eutectoid response, in which austenite decomposes into alternating layers of ferrite and cementite. This lamellar shape consists of thin, alternating layers of those stages, which shape because of the eutectoid transformation.
The formation of pearlite starts offevolved whilst austenite, that is a unmarried-segment stable answer of carbon in iron, reaches the eutectoid temperature and starts offevolved to cool. As the temperature drops, the austenite turns into risky and begins offevolved to decompose. This decomposition outcomes withinside the formation of pearlite, in which the cementite precipitates as first-rate layers in the ferrite matrix. The lamellar shape of pearlite is vital as it offers a stability among electricity and ductility.
Effect of Cooling Rates on Microstructure
The cooling price at some stage in the eutectoid response has a considerable impact at the ensuing microstructure of the metallic:
Slow Cooling:
When metallic is cooled slowly via the eutectoid temperature, pearlite paperwork with well-described lamellar structures. Slow cooling permits for the formation of coarse pearlite, in which the alternating layers of ferrite and cementite are thicker. Coarse pearlite offers exact ductility and effect resistance however might also additionally have decrease electricity in comparison to finer pearlite.
Moderate Cooling:
If the cooling price is moderate, pearlite paperwork with a finer lamellar shape. This finer pearlite gives progressed electricity and hardness at the same time as nonetheless preserving affordable ductility. The first-rate shape is useful for packages requiring a stability among electricity and toughness.
Rapid Cooling:
Rapid cooling or quenching can save you the formation of pearlite and as a substitute cause the formation of martensite, a miles tougher and greater brittle segment. This takes place due to the fact the austenite does now no longer have sufficient time to decompose into pearlite, ensuing in a supersaturated shape. Rapid cooling also can cause the formation of bainite if the cooling price is managed to keep away from the formation of martensite.
The Eutectic Reaction and Cast Irons in Iron Carbon Diagram
Definition and Characteristics
The eutectic response withinside the iron-carbon gadget happens at a selected composition and temperature, wherein a liquid segment solidifies into specific stable stages simultaneously. For iron-carbon alloys, this response takes place at about 1147°C with a carbon content material of approximately 4.3%. During this process, the liquid iron-cementite alloy solidifies right into a aggregate of austenite and cementite. This eutectic response is critical withinside the formation of numerous styles of solid iron and dictates their microstructural characteristics.
Types of Cast Irons: White, Gray, Ductile
White Cast Iron:
White solid iron is characterised through its tough and brittle nature because of the presence of cementite (Fe₃C) because the number one microstructural component. It bureaucracy whilst the eutectic response happens rapidly, stopping the formation of graphite. The ensuing shape includes a matrix of cementite with a tough, white look whilst fractured, for this reason the name “white solid iron.” It is regularly utilized in programs requiring excessive put on resistance, inclusive of in crushers and mill liners.
Gray Cast Iron:
Gray solid iron is prominent through its graphite flakes or nodules, which can be shaped at some point of the eutectic solidification process. The graphite imparts a grey colour to the fracture floor and gives advanced machinability and ductility in comparison to white solid iron. The presence of graphite additionally complements the material`s capacity to soak up vibrations. Gray solid iron is generally utilized in engine blocks, pipes, and device parts.
Ductile Cast Iron (Nodular Cast Iron):
Ductile solid iron, additionally referred to as nodular solid iron, includes graphite withinside the shape of rounded nodules as opposed to flakes. This nodular shape is performed through including small quantities of factors inclusive of magnesium to the molten iron earlier than casting. The nodular graphite shape gives advanced mechanical properties, along with elevated electricity, ductility, and toughness. Ductile solid iron is utilized in programs requiring excessive electricity and effect resistance, inclusive of automobile additives and heavy machinery.
Eutectic Composition and Solidification
Eutectic Composition:
The eutectic composition withinside the iron-carbon gadget is about 4.3% carbon. At this composition, the liquid alloy solidifies right into a aggregate of austenite and cementite. This composition is critical in solid iron manufacturing because it determines the form of solid iron so one can shape primarily based totally at the cooling fee and solidification conditions.
Solidification:
During solidification, the liquid iron-cementite alloy undergoes the eutectic response to shape a dual-segment microstructure. As the alloy cools, it first reaches the eutectic temperature, wherein the liquid transforms right into a aggregate of austenite and cementite. The unique microstructure, whether or not it’s white solid iron, grey solid iron, or ductile solid iron, relies upon on elements inclusive of cooling fee, alloy composition, and the presence of alloying factors.
The Peritectic Reaction in Steel Making in Iron Carbon Diagram
Definition and Process
The peritectic response is a key segment transformation withinside the iron-carbon machine that takes place at a selected temperature and composition. In steelmaking, the peritectic response includes the interplay among stable stages and a liquid segment to shape a brand new stable segment. Specifically, it takes place at about 1495°C and a carbon content material of round 0.18%.
During the peritectic response, the subsequent takes place:
Liquid Iron (L) and Ferrite (α-Fe): At excessive temperatures, a liquid iron-carbon alloy reacts with ferrite to shape austenite (γ-Fe).
Formation of Austenite (γ-Fe): As the liquid and ferrite interact, the austenite segment forms, that is solid on the temperature wherein the peritectic response takes place. This response is vast withinside the solidification and cooling of positive metal grades.
The peritectic response is much less not unusualplace in sensible steelmaking in comparison to different reactions just like the eutectoid, however it nonetheless performs a function withinside the formation of particular metal structures.
Impact on Steel Properties
The peritectic response affects metal residences in numerous ways:
Microstructure: The formation of austenite in the course of the peritectic response can have an effect on the ensuing microstructure of the metal. The presence of austenite impacts the distribution and length of different stages, which affects the general cloth residences.
Strength and Ductility: Steel with a microstructure encouraged through the peritectic response can showcase one-of-a-kind mechanical residences in comparison to different steels. The austenite segment shaped in the course of this response can have an effect on the power and ductility of the metal, relying on how it’s far in addition processed and heat-treated.
Control in Industrial Processes
In commercial steelmaking, controlling the peritectic response includes cautious control of temperature and composition to attain the favored metal residences:
Temperature Management: Maintaining the best temperature in the course of solidification is vital for controlling the peritectic response. The response commonly takes place at excessive temperatures, so specific temperature manipulate is important to make certain the formation of austenite and keep away from undesirable segment transformations.
Alloy Composition: The composition of the iron-carbon alloy influences the peritectic response. Adjusting the carbon content material and different alloying factors facilitates manipulate the response and the ensuing microstructure. This permits for the tailoring of metal residences for particular applications.
Heat Treatment of Steel in Iron Carbon Diagram
Overview of Heat Treatment Processes
Heat remedy approaches normally contain numerous stages:
Heating: Steel is heated to a particular temperature to acquire the favored section transformations.
Holding: The metallic is held on the goal temperature for a described duration to make sure uniform transformation.
Cooling: The metallic is cooled at a managed fee to steer the formation of various microstructures.
Annealing, Normalizing, and Quenching
Annealing:
Annealing entails heating metallic to a temperature wherein its shape will become greater uniform, accompanied via way of means of gradual cooling. This manner facilitates relieve inner stresses, lessen hardness, and enhance ductility and toughness. Annealing
commonly happens in numerous stages:
Full Annealing: The metallic is heated above its essential temperature, held there to permit entire transformation to austenite, after which slowly cooled to shape a soft, uniform microstructure.
Process Annealing: Performed at a decrease temperature than complete annealing, it’s far used to melt metallic that has been work-hardened throughout production approaches.
Spheroidizing: This is a specialised shape of annealing used to transform cementite in metallic to round particles, enhancing machinability.
Normalizing:
Normalizing entails heating metallic to a temperature above the essential range, then cooling it in air. This manner refines the grain shape, main to advanced mechanical houses and consistency. Normalizing:
Improves Mechanical Properties: It produces a greater uniform grain length and eliminates the results of preceding warmness remedies or mechanical work.
Enhances Ductility: The resultant microstructure, frequently pearlite with a first-class ferrite shape, offers a very good stability of energy and ductility.
Quenching:
Quenching is a fast cooling manner used to harden metallic. It entails heating metallic to a temperature wherein it will become austenitic after which fast cooling it, normally in water, oil, or brine. This fast cooling transforms the austenite into martensite, a tough and brittle section. Quenching is commonly accompanied via way of means of:
High Hardness: The ensuing martensite section offers excessive hardness and put on resistance.
Internal Stresses: The fast cooling can set off inner stresses and brittleness, that is why it’s far frequently accompanied via way of means of tempering.
Tempering and its Effects
Tempering:
Tempering is a post-quenching warmness remedy manner designed to lessen brittleness and enhance toughness. It entails reheating the quenched metallic to a temperature under the eutectoid temperature, retaining it for a duration, after which cooling it. The key results of tempering include:
Reduction of Brittleness: Tempering relieves inner stresses and decreases the brittleness because of the martensitic transformation.
Improved Toughness: By adjusting the tempering temperature and time, the metallic can acquire a stability among hardness and toughness, making it appropriate for diverse applications.
Microstructural Changes: Tempering ends in the formation of tempered martensite, wherein a number of the extra carbon in martensite precipitates out as carbide particles, ensuing in a greater solid microstructure.
Transformation Iron Carbon Diagram: TTT and CCT
Time-Temperature-Transformation (TTT) Diagrams
Structure of TTT Diagrams:
TTT diagrams plot temperature at the vertical axis and time at the horizontal axis. They usually function numerous crucial lines:
Austenite to Pearlite: Shows the time and temperature at which austenite transforms into pearlite.
Austenite to Bainite: Indicates the transformation of austenite to bainite at numerous temperatures.
Martensite Start (Ms) and Martensite Finish (Mf): Mark the temperatures at which austenite starts offevolved and completes transformation into martensite.
Key Concepts:
Critical Cooling Rate: The minimal charge of cooling required to keep away from the formation of pearlite and gain a martensitic structure.
Transformation Stages: The diagram enables are expecting which stages will shape throughout unique levels of cooling and holding.
Applications:
TTT diagrams are used to layout warmth remedy approaches and are expecting the ensuing microstructures primarily based totally on precise cooling schedules. They are essential for attaining preferred mechanical residences in metal with the aid of using controlling the section ameliorations throughout cooling.
Continuous Cooling Transformation (CCT) Diagrams
Structure of CCT Diagrams:
CCT diagrams plot temperature towards cooling charge (frequently on a logarithmic scale). They display how the transformation of stages like pearlite, bainite, and martensite varies with the charge of cooling.
Key Concepts:
Continuous Cooling Curves: The diagram shows how unique stages shape as metal is cooled at numerous fees. For example, slower cooling fees may cause pearlite formation, whilst quicker fees may produce martensite.
Transformation Zones: The diagram highlights zones wherein unique ameliorations arise, taking into account predictions approximately which stages will shape beneathneath precise cooling situations.
Applications:
CCT diagrams are used to tailor cooling fees in commercial approaches to gain preferred microstructures. They are crucial for controlling the residences of metal throughout approaches like quenching and tempering, supporting to optimize overall performance and save you defects.
Applications and Interpretation
Designing Heat Treatments:
Both TTT and CCT diagrams are used to layout warmth remedy approaches with the aid of using predicting the ensuing microstructures primarily based totally on temperature and cooling fees. This permits engineers to specify the precise situations had to gain the preferred mechanical residences in metal.
Predicting Microstructure:
By decoding those diagrams, you can still are expecting the microstructural modifications a good way to arise beneathneath unique warmth remedy situations. For instance, a fast cooling charge on a CCT diagram may display the formation of martensite, whilst a slower charge may bring about pearlite or bainite.
Optimizing Material Properties:
Understanding TTT and CCT diagrams enables in optimizing fabric residences for precise applications. For example, attaining a stability among energy and sturdiness may be executed with the aid of using deciding on suitable cooling fees and temperatures primarily based totally at the diagrams.
Preventing Defects:
Proper interpretation and alertness of those diagrams assist in stopping defects together with warping, cracking, or unwanted section formations. This guarantees the reliability and overall performance of metal additives of their meant applications.
Influence of Alloying Elements in Iron Carbon Diagram
Role of Carbon and Other Elements
Carbon:
Carbon is the number one alloying detail in metal and has a profound effect on its homes. It will increase hardness and electricity through forming stable answers with iron and contributing to the formation of cementite (Fe₃C). The quantity of carbon determines the metal`s type and its mechanical homes:
Low Carbon Steels (0.6% C): These are tougher and greater put on-resistant, perfect for tools, blades, and excessive-electricity components.
Alloying Elements:
Various alloying factors are brought to metal to beautify particular homes:
Chromium (Cr): Improves hardness, electricity, and corrosion resistance. It is a key thing in stainless steels and contributes to the formation of chromium carbide, which complements put on resistance.
Nickel (Ni): Increases durability and effect resistance, specially at low temperatures. It is regularly utilized in mixture with chromium in stainless steels.
Molybdenum (Mo): Enhances hardenability and electricity, and improves resistance to excessive temperatures and corrosion. It is usually utilized in device steels and excessive-electricity low-alloy steels.
Vanadium (V): Refines the grain shape and improves electricity and put on resistance. It facilitates in forming vanadium carbide, which strengthens the metal.
Manganese (Mn): Improves hardenability and electricity. It additionally facilitates in eliminating sulfur, that may motive brittleness.
Silicon (Si): Used as a deoxidizer and to enhance electricity and magnetic homes in electric steels.
Frequently Asked Questions (FAQs)
- What are alloying elements in steel?
Alloying elements are additional substances mixed with steel to enhance its properties. Common alloying elements include carbon, chromium, nickel, molybdenum, vanadium, manganese, and silicon. Each of these elements affects the steel’s characteristics in different ways, such as improving its strength, hardness, corrosion resistance, or toughness.
2. How does carbon affect the properties of steel?
Carbon is a crucial alloying element in steel and has a significant impact on its properties. In low carbon steels (less than 0.3% carbon), the material is softer and more ductile, making it easier to weld. Medium carbon steels (between 0.3% and 0.6% carbon) offer a balance between strength and ductility, which is suitable for structural applications. High carbon steels (more than 0.6% carbon) are harder and more wear-resistant but less ductile, making them ideal for tools and high-strength components.
3. What role does chromium play in steel?
Chromium enhances several key properties of steel. It increases hardness and tensile strength and significantly improves corrosion resistance, making it an essential component in stainless steels. Additionally, chromium forms chromium carbides within the steel, which improves wear resistance and helps maintain the steel’s structural integrity under harsh conditions.
3. How does nickel affect steel?
Nickel improves the toughness of steel, especially at low temperatures, which enhances its impact resistance. It also contributes to corrosion resistance and is often combined with chromium in stainless steels to provide enhanced protection against oxidation and rust. Nickel also helps stabilize the austenitic structure, which can improve the steel’s overall performance.
4. What is the impact of molybdenum on steel?
Molybdenum plays a critical role in improving steel’s hardness and strength. It increases the steel’s hardenability and stability at high temperatures, which helps maintain the material’s strength under extreme conditions. Molybdenum also improves resistance to corrosion and oxidation, making it valuable for specific high-strength applications.
5. How does vanadium influence steel?
Vanadium is used to refine the grain structure of steel, leading to improved mechanical properties. It helps form vanadium carbides, which enhance the steel’s strength and wear resistance. By fine-tuning the microstructure, vanadium contributes to a more durable and robust material.
What does manganese do in steel?






